NEWS AS ON Wednesday, 18 December 2013
Wednesday, 18 December 2013
DNA Motor 'Walks' Along Nanotube, Transports Tiny Particle
DNA Motor 'Walks' Along Nanotube, Transports Tiny Particle
Dec. 17, 2013 — Researchers
have created a new type of molecular motor made of DNA and demonstrated
its potential by using it to transport a nanoparticle along the length
of a carbon nanotube.
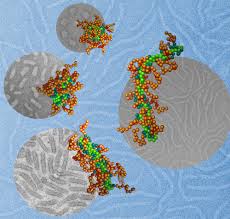
This
illustration depicts the walking mechanism of a new type of DNA motor
that researchers have demonstrated by using it to transport a
nanoparticle along the length of a carbon nanotube. (Credit: Purdue
University image/Tae-Gon Cha)
The design was inspired by natural biological motors that have
evolved to perform specific tasks critical to the function of cells,
said Jong Hyun Choi, a Purdue University assistant professor of
mechanical engineering.
Whereas biological motors are made of protein, researchers are trying to create synthetic motors based on DNA, the genetic materials in cells that consist of a sequence of four chemical bases: adenine, guanine, cytosine and thymine. The walking mechanism of the synthetic motors is far slower than the mobility of natural motors. However, the natural motors cannot be controlled, and they don't function outside their natural environment, whereas DNA-based motors are more stable and might be switched on and off, Choi said.
"We are in the very early stages of developing these kinds of synthetic molecular motors," he said.
New findings were detailed in a research paper published this month in the journal Nature Nanotechnology.
In coming decades, such molecular motors might find uses in drug delivery, manufacturing and chemical processing.
The new motor has a core and two arms made of DNA, one above and one below the core. As it moves along a carbon-nanotube track it continuously harvests energy from strands of RNA, molecules vital to a variety of roles in living cells and viruses.
The Nature Nanotechnology paper was authored by graduate students Tae-Gon Cha, Jing Pan and Haorong Chen; former undergraduate student Janette Salgado; graduate student Xiang Li; Chengde Mao, a professor of chemistry; and Choi.
"Our motors extract chemical energy from RNA molecules decorated on the nanotubes and use that energy to fuel autonomous walking along the carbon nanotube track," Choi said.
The core is made of an enzyme that cleaves off part of a strand of RNA. After cleavage, the upper DNA arm moves forward, binding with the next strand of RNA, and then the rest of the DNA follows. The process repeats until reaching the end of the nanotube track.
Researchers used the motor to move nanoparticles of cadmium disulfide along the length of a nanotube. The nanoparticle is about 4 nanometers in diameter.
The researchers combined two fluorescent imaging systems to document the motor's movement, one in the visible spectrum and the other in the near-infrared range. The nanoparticle is fluorescent in visible light and the nanotubes are fluorescent in the near-infrared.
The motor took about 20 hours to reach the end of the nanotube, which was several microns long, but the process might be sped up by changing temperature and pH, a measure of acidity.
This work has been supported by the U.S. Office of Naval Research.
Whereas biological motors are made of protein, researchers are trying to create synthetic motors based on DNA, the genetic materials in cells that consist of a sequence of four chemical bases: adenine, guanine, cytosine and thymine. The walking mechanism of the synthetic motors is far slower than the mobility of natural motors. However, the natural motors cannot be controlled, and they don't function outside their natural environment, whereas DNA-based motors are more stable and might be switched on and off, Choi said.
"We are in the very early stages of developing these kinds of synthetic molecular motors," he said.
New findings were detailed in a research paper published this month in the journal Nature Nanotechnology.
In coming decades, such molecular motors might find uses in drug delivery, manufacturing and chemical processing.
The new motor has a core and two arms made of DNA, one above and one below the core. As it moves along a carbon-nanotube track it continuously harvests energy from strands of RNA, molecules vital to a variety of roles in living cells and viruses.
The Nature Nanotechnology paper was authored by graduate students Tae-Gon Cha, Jing Pan and Haorong Chen; former undergraduate student Janette Salgado; graduate student Xiang Li; Chengde Mao, a professor of chemistry; and Choi.
"Our motors extract chemical energy from RNA molecules decorated on the nanotubes and use that energy to fuel autonomous walking along the carbon nanotube track," Choi said.
The core is made of an enzyme that cleaves off part of a strand of RNA. After cleavage, the upper DNA arm moves forward, binding with the next strand of RNA, and then the rest of the DNA follows. The process repeats until reaching the end of the nanotube track.
Researchers used the motor to move nanoparticles of cadmium disulfide along the length of a nanotube. The nanoparticle is about 4 nanometers in diameter.
The researchers combined two fluorescent imaging systems to document the motor's movement, one in the visible spectrum and the other in the near-infrared range. The nanoparticle is fluorescent in visible light and the nanotubes are fluorescent in the near-infrared.
The motor took about 20 hours to reach the end of the nanotube, which was several microns long, but the process might be sped up by changing temperature and pH, a measure of acidity.
This work has been supported by the U.S. Office of Naval Research.
Tuesday, 17 December 2013
Noble Gas Molecule Discovered in Space
Noble Gas Molecule Discovered in Space
Dec. 12, 2013 — A molecule containing a noble gas has been discovered in space by a team including astronomers from Cardiff University.

In blue,
visible light from the Crab Nebula seen by the Hubble Space Telescope.
This comes from emissions of gases in the nebula, which are energised by
the neutron star at the centre. In red, far infrared light seen by the
Herschel Space Observatory. This comes mainly from cold dust and gas.
(Credit: NASA, ESA, Alison Loll & Jeff Hester (University of
Arizona))
The find was made using a Cardiff-led instrument aboard Europe's
Herschel Space Observatory. The molecule, argon hydride, was seen in the
Crab Nebula, the remains of a star that exploded 1,000 years ago.
Before the discovery, molecules of this kind have only been studied in
laboratories on Earth.
The noble gases, which include helium, argon, radon and krypton, usually do not react easily with other chemical elements, and are often found on their own. In the right circumstances, however, they can form molecules with other elements. Such chemical compounds have only ever been studied in laboratories on Earth, leading astronomers to assume the right conditions simply do not occur in space.
"The Crab Nebula was only formed 1000 years ago when a massive star exploded," said Dr Haley Gomez of Cardiff University's School of Physics and Astronomy. "Not only is it very young in astronomical terms, but also relatively close, at just 6,500 light years away, providing an excellent way to study what happens in these stellar explosions. Last year, we used the European Space Agency's Herschel Space Observatory to study the intricate network of gas filaments to show how exploding stars are creating huge amounts of space dust."
Further measurements of the Crab Nebula were made using Herschel's SPIRE instrument. Its development and operation was led by Professor Matt Griffin, from the School of Physics and Astronomy. As molecules spin in space, they emit light of very specific wavelengths, or colours, called "emission lines." The precise wavelength is dictated by the composition and structure of the molecule. Studying the emission lines observed by the SPIRE instrument allows astronomers to study the chemistry of outer space.
The team, led by Professor Mike Barlow from University College London, did not set out to make the discovery, but stumbled upon it almost by accident. "We were really concentrating on studying the dust in the filaments with SPIRE, and out pops these two bright emission lines exactly where we see the dust shining," says Dr Gomez. "The team had a hard time figuring out what these lines were from, as no-one had seen them before."
Professor Barlow said, "At first, the discovery of argon seemed bizarre. With hot gas still expanding at high speeds after the explosion, a supernova remnant is a harsh, hot and hostile environment, and one of the places where we least expected to find a noble-gas based molecule."
It now seems the Crab Nebula provides exactly the right conditions to form such molecules. The argon was produced in the initial stellar explosion, and then ionised, or energised, with electrons stripped from the atoms in resulting intense radiation as shockwaves. These shockwaves led to the formation of the network of cool filaments containing cold molecular hydrogen, made of two hydrogen atoms. The ionised argon then mixed with the cool gas to provide perfect conditions for noble gas compounds to form.
The measurements allowed the team to gauge other properties in argon molecules. "Finding this kind of molecule allowed us to evaluate the type (or isotope) of argon we discovered in the Crab Nebula," said Dr Gomez. "We now know that it is different from argon we see in rocks on the Earth. Future measurements will allow us to probe what exactly took place in the explosion 1000 years ago."
"What a great detective story," added Prof Matt Griffin, from Cardiff University, and lead scientist of the team behind the SPIRE instrument. "Here we see the excellent performance of the Herschel-SPIRE spectrometer, the expertise of the instrument team in producing the highest quality data, and the tenacity and vision of the scientists analysing it, all coming together to make an intriguing new discovery."
The noble gases, which include helium, argon, radon and krypton, usually do not react easily with other chemical elements, and are often found on their own. In the right circumstances, however, they can form molecules with other elements. Such chemical compounds have only ever been studied in laboratories on Earth, leading astronomers to assume the right conditions simply do not occur in space.
"The Crab Nebula was only formed 1000 years ago when a massive star exploded," said Dr Haley Gomez of Cardiff University's School of Physics and Astronomy. "Not only is it very young in astronomical terms, but also relatively close, at just 6,500 light years away, providing an excellent way to study what happens in these stellar explosions. Last year, we used the European Space Agency's Herschel Space Observatory to study the intricate network of gas filaments to show how exploding stars are creating huge amounts of space dust."
Further measurements of the Crab Nebula were made using Herschel's SPIRE instrument. Its development and operation was led by Professor Matt Griffin, from the School of Physics and Astronomy. As molecules spin in space, they emit light of very specific wavelengths, or colours, called "emission lines." The precise wavelength is dictated by the composition and structure of the molecule. Studying the emission lines observed by the SPIRE instrument allows astronomers to study the chemistry of outer space.
The team, led by Professor Mike Barlow from University College London, did not set out to make the discovery, but stumbled upon it almost by accident. "We were really concentrating on studying the dust in the filaments with SPIRE, and out pops these two bright emission lines exactly where we see the dust shining," says Dr Gomez. "The team had a hard time figuring out what these lines were from, as no-one had seen them before."
Professor Barlow said, "At first, the discovery of argon seemed bizarre. With hot gas still expanding at high speeds after the explosion, a supernova remnant is a harsh, hot and hostile environment, and one of the places where we least expected to find a noble-gas based molecule."
It now seems the Crab Nebula provides exactly the right conditions to form such molecules. The argon was produced in the initial stellar explosion, and then ionised, or energised, with electrons stripped from the atoms in resulting intense radiation as shockwaves. These shockwaves led to the formation of the network of cool filaments containing cold molecular hydrogen, made of two hydrogen atoms. The ionised argon then mixed with the cool gas to provide perfect conditions for noble gas compounds to form.
The measurements allowed the team to gauge other properties in argon molecules. "Finding this kind of molecule allowed us to evaluate the type (or isotope) of argon we discovered in the Crab Nebula," said Dr Gomez. "We now know that it is different from argon we see in rocks on the Earth. Future measurements will allow us to probe what exactly took place in the explosion 1000 years ago."
"What a great detective story," added Prof Matt Griffin, from Cardiff University, and lead scientist of the team behind the SPIRE instrument. "Here we see the excellent performance of the Herschel-SPIRE spectrometer, the expertise of the instrument team in producing the highest quality data, and the tenacity and vision of the scientists analysing it, all coming together to make an intriguing new discovery."
Monday, 16 December 2013
Researchers Split Water Into Hydrogen, Oxygen Using Light, Nanoparticles
Researchers Split Water Into Hydrogen, Oxygen Using Light, Nanoparticles
Dec. 15, 2013 — Researchers
from the University of Houston have found a catalyst that can quickly
generate hydrogen from water using sunlight, potentially creating a
clean and renewable source of energy.

Water. Researchers have used cobalt oxide nanoparticles to split water into hydrogen and oxygen. (Credit: © gertrudda / Fotolia)
Their research, published online Sunday in Nature Nanotechnology, involved the use of cobalt oxide nanoparticles to split water into hydrogen and oxygen.
Jiming Bao, lead author of the paper and an assistant professor in the Department of Electrical and Computer Engineering at UH, said the research discovered a new photocatalyst and demonstrated the potential of nanotechnology in engineering a material's property, although more work remains to be done.
Bao said photocatalytic water-splitting experiments have been tried since the 1970s, but this was the first to use cobalt oxide and the first to use neutral water under visible light at a high energy conversion efficiency without co-catalysts or sacrificial chemicals. The project involved researchers from UH, along with those from Sam Houston State University, the Chinese Academy of Sciences, Texas State University, Carl Zeiss Microscopy LLC, and Sichuan University.
Researchers prepared the nanoparticles in two ways, using femtosecond laser ablation and through mechanical ball milling. Despite some differences, Bao said both worked equally well.
Different sources of light were used, ranging from a laser to white light simulating the solar spectrum. He said he would expect the reaction to work equally well using natural sunlight.
Once the nanoparticles are added and light applied, the water separates into hydrogen and oxygen almost immediately, producing twice as much hydrogen as oxygen, as expected from the 2:1 hydrogen to oxygen ratio in H2O water molecules, Bao said.
The experiment has potential as a source of renewable fuel, but at a solar-to-hydrogen efficiency rate of around 5 percent, the conversion rate is still too low to be commercially viable. Bao suggested a more feasible efficiency rate would be about 10 percent, meaning that 10 percent of the incident solar energy will be converted to hydrogen chemical energy by the process.
Other issues remain to be resolved, as well, including reducing costs and extending the lifespan of cobalt oxide nanoparticles, which the researchers found became deactivated after about an hour of reaction.
"It degrades too quickly," said Bao, who also has appointments in materials engineering and the Department of Chemistry.
The work, supported by the Welch Foundation, will lead to future research, he said, including the question of why cobalt oxide nanoparticles have such a short lifespan, and questions involving chemical and electronic properties of the material.
Jiming Bao, lead author of the paper and an assistant professor in the Department of Electrical and Computer Engineering at UH, said the research discovered a new photocatalyst and demonstrated the potential of nanotechnology in engineering a material's property, although more work remains to be done.
Bao said photocatalytic water-splitting experiments have been tried since the 1970s, but this was the first to use cobalt oxide and the first to use neutral water under visible light at a high energy conversion efficiency without co-catalysts or sacrificial chemicals. The project involved researchers from UH, along with those from Sam Houston State University, the Chinese Academy of Sciences, Texas State University, Carl Zeiss Microscopy LLC, and Sichuan University.
Researchers prepared the nanoparticles in two ways, using femtosecond laser ablation and through mechanical ball milling. Despite some differences, Bao said both worked equally well.
Different sources of light were used, ranging from a laser to white light simulating the solar spectrum. He said he would expect the reaction to work equally well using natural sunlight.
Once the nanoparticles are added and light applied, the water separates into hydrogen and oxygen almost immediately, producing twice as much hydrogen as oxygen, as expected from the 2:1 hydrogen to oxygen ratio in H2O water molecules, Bao said.
The experiment has potential as a source of renewable fuel, but at a solar-to-hydrogen efficiency rate of around 5 percent, the conversion rate is still too low to be commercially viable. Bao suggested a more feasible efficiency rate would be about 10 percent, meaning that 10 percent of the incident solar energy will be converted to hydrogen chemical energy by the process.
Other issues remain to be resolved, as well, including reducing costs and extending the lifespan of cobalt oxide nanoparticles, which the researchers found became deactivated after about an hour of reaction.
"It degrades too quickly," said Bao, who also has appointments in materials engineering and the Department of Chemistry.
The work, supported by the Welch Foundation, will lead to future research, he said, including the question of why cobalt oxide nanoparticles have such a short lifespan, and questions involving chemical and electronic properties of the material.












0 comments:
Post a Comment