Molecular Machine Could Hold Key to More Efficient Manufacturing
Molecular Machine Could Hold Key to More Efficient Manufacturing

08:06 Kalyan Gupta 0 Comments

07:25 Kalyan Gupta 0 Comments
07:23 Kalyan Gupta 0 Comments
03:03 Kalyan Gupta 0 Comments

06:14 Kalyan Gupta 0 Comments

06:13 Kalyan Gupta 0 Comments
09:13 Kalyan Gupta 0 Comments
09:12 Kalyan Gupta 0 Comments
08:44 Kalyan Gupta 0 Comments
Our bodies are full of molecular machines and their synthetic counterparts may soon be all around us too
© SCIENCE PHOTO LIBRARY
|
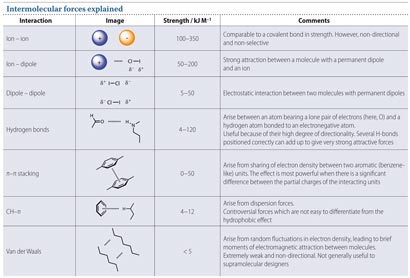
Intermolecular forces explained. Download the pdf at the end of the article to see a high resolution version of this image.
|
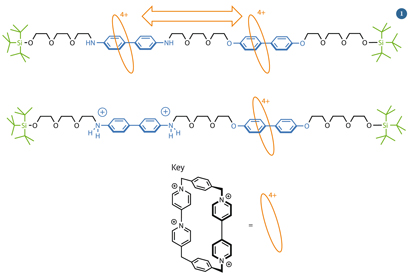
Fig 1 - The key parts of Stoddart's molecular shuttle are the two stations (blue), the shuttle (orange) and the bulky stoppers (green)
|
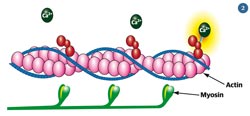
Fig 2 - The actin and myosin filaments in muscles use protein-based machines to pull past each other
|
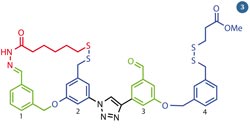
Fig 3 - Leigh's molecular walker can take steps along a pathway of alternating functional groups
|
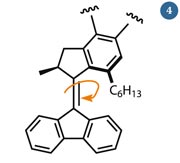
Fig 4 - Feringa's molecular rotor is powered by electrons, which excite the double bond and induce an E/Z-isomerisation
|
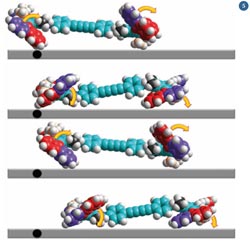
Fig 5 - If the four rotors work in unison, the nanocar begins to drive along the surface
|
08:35 Kalyan Gupta 0 Comments
08:32 Kalyan Gupta 0 Comments
02:41 Kalyan Gupta 0 Comments
02:39 Kalyan Gupta 0 Comments
02:34 Kalyan Gupta 0 Comments
 Nokia may not be too funky with their mobile phone designs for mass production but you have to admit, their concept designs are something that look, quite literally, out of this world. Morph, a joint Nanotechnology concept, developed by Nokia Research Center (NRC) and the University of Cambridge (UK) – was launched today alongside the “Design and the Elastic Mind” exhibition, at The Museum of Modern Art (MoMA) in New York. Morph features in both the exhibition catalog and on MoMA’s official website.
Nokia may not be too funky with their mobile phone designs for mass production but you have to admit, their concept designs are something that look, quite literally, out of this world. Morph, a joint Nanotechnology concept, developed by Nokia Research Center (NRC) and the University of Cambridge (UK) – was launched today alongside the “Design and the Elastic Mind” exhibition, at The Museum of Modern Art (MoMA) in New York. Morph features in both the exhibition catalog and on MoMA’s official website.

09:21 Kalyan Gupta 0 Comments
Background | ||||||
Since its inception, Strem has focused on offering unique organometallic compounds for both academic and industrial research purposes. Close relationships with leading researchers in the field have enabled Strem to stay abreast of the latest scientific advances in and regularly add novel chemicals to our product portfolio. Most recently, Strem has embraced the emerging area of nanotechnology and formed a collaboration with the Max-Planck-Institut fuer Kohlenforschung. A series of nanomaterials, including including metal nanoclusters, metal nanocolloids (organosols and hydrosols), metal nanopowders, metal nanoparticles, and magnetic fluids are now available from Strem. Below is a discussion of potential applications for magnetic fluids.
Magnetic Fluids
Magnetic fluids or ferrofluids are stable colloidal suspensions of magnetic metal nanoparticles in a carrier liquid. To maintain suspension, the particles are coated with a surface active layer. The carrier liquid can be a hydrocarbon, ester, perfluorether, water, etc. Properties of nanomagnetic particles, films and bulk materials include exchange coupling, tunneling magnetoresistance, giant magnetoresistance, single domain behavior, and superparamagnetism. Magnetite based magnetic fluids do not have sufficient magnetic properties for many applications. Colloidal nanosized metallic Fe, Co or Fe/Co alloys like those offered by Strem do posses ideal properties.
Separations
Magnetic fluids are attractive in separation applications because the reactivity of the particles can be tailored by modifying their surface coatings, they afford very high surface areas without the use of porous absorbents, and they can be recovered for reuse.
Using Magnetic Nanoparticles in Nanocatalysts - Processing Methods and Benefits
Nanocatalysts containing magnetic nanoparticles are being developed. Silica and carbon are utilized to maintain the stability of the nanoparticles under reaction conditions. Functionalized surfaces on the nanoparticles include immobilizing sites for catalytically active species such as nanometal particles, enzymes, and homogenous catalysts. The catalysts are easily separated by utilizing the magnetic interaction between the magnetic nanoparticle and an external applied magnetic field. These hybrid catalysts offer the advantages of homogeneous and heterogeneous catalysis combined
Using Magnetic Nanomaterials in Filters and to Remove Sulfur Compounds from Hydrocarbon Fuels
Magnetic nanomaterials have also been used in filters to remove selected impurities from various types of fluids. The separation of organic contaminants such as polyaromatic hydrocarbons from water and the removal of sulfur compounds from hydrocarbon fuels, are also being investigated with magnetic fluids.
Current Research into Biochemical Reactions Using Magnetic Nanoparticles
In biochemical reactions, magnetic nanoparticles are being investigated as a means to aid in the separation and recovery of target biomolecules such as DNA, RNA, and proteins. Non-ferrous metals can be rapidly recovered from solid wastes using sink-float separation, which utilized the magnetic fluid as a coating on the materials. Metals of different densities can be separated by controlling the field gradient, which levitates the materials.
Magnetic Media
Magnetic nanoparticles have significant potential for increasing magnetic storage capabilities. Cobalt and Pt/Co bimetallic nanoparticles are being investigated as magnetic storage devices. Researchers are studying core-shell cobalt nanoparticles embedded in an anti-ferromagnetic matrix. Cobalt nanoparticles have also been found to self-assemble into ‘nanorings’ that can store magnetic information at room temperature. These magnetic rings are being considered as memory elements in devices for long-term data storage and magnetic random-access memory (MRAM).
How Magnetic Cobalt Nanocrystals Can Improve Magnetic Random-Access Memory (MRAM) Systems
MRAM systems are currently severely under-damped systems. Just a few monolayers of magnetic cobalt nanocrystals deposited on the surface of the magnetic layer in an MRAM device have been found to provide a tripling of the dampening effect. In addition, a 2-2-3 fold enhancement of the magnetic field can be achieved in an MRAM device when a superparamagnetic nanoparticle layer is used in place of the continuous liner. As a result, a third of the current can be used to obtain the same magnetic field as before. Alternatively, the same amount of current can be used to obtain a much more thermally stable system.
Industry Applications for Polystyrene Films Containing Gold Nanoparticles
Polystyrene films containing gold nanoparticles have been found to store a charge and act as organic memory devices. The polystyrene base, which is carried in a liquid, can be applied through spray, paint, or print technology. 3-D arrays can also be constructed for high density storage and could have applications in digital memory chips for computers, digital cameras, and cell phones. The goal of other researchers is to have each nanomagnetic particle support one bit of digital information.
Industrial Applications for Magnetic Fluids
Industrial applications for magnetic fluids include seals, dampers, and moving coil loud speakers. In seals, the ferrofluid acts as a magnetic O-ring and provides hermetic sealing with zero leakage. Because the fluid is also lubricating, minimal wear occurs as well. Seals based on magnetic fluids have been developed for vacuum systems, gas-air media, and liquids (water, oil, and chemically active media). They find use in high speed computer disk drives, rotating joints in clean rooms, and in industrial applications where control of volatile emissions and protection of sensitive environments is important.
Using Magnetic Fluids as Dampers - Processing Methods
Magnetic fluids can change their apparent viscosity in proportion to the strength of an applied magnetic field. Therefore, the viscosity can be controlled dynamically, which allows for active damping. Large amounts of mechanical power can be controlled with a small amount of electrical power, making this method of vibration control much more efficient than traditional systems. It is possible to adjust the stiffness thousands of times per second. As dampers, magnetic fluids are highly efficient over a wide frequency and temperature range. They are used to dampen automotive devices, space structures, wing oscillations in aircraft, protect devices and some buildings from vibrational damage, and to improve comfort during transport.
Using Magnetic Fluids in Moving Coil Loudspeakers - Processes and Benefits
In a moving coil loudspeaker, the magnetic fluid conducts heat away from the voice coil, keeps the voice coil concentric with the magnet, and passively dampens movement of the cone. The magnetic fluid is placed in what is normally the air gap around the voice coil. Advantages of using magnetic fluids in moving coil loudspeakers include increased power handling, a smooth frequency response curve, and reduced distortion.
List of Nanomaterials that Strem Chemicals Can Supply to All Types of Industry
Numerous other potential applications exist for magnetic nanomaterials from Strem. Areas currently under investigation include non-destructive testing, finishing polishing, domain observation, telecommunications, magnetic inks, robotics, automotive, surface analysis, and medical/pharmaceutical such as targeted drug delivery, diagnostics, and biosensors. A listing of specific including metal nanoclusters, metal nanocolloids (organosols and hydrosols), metal nanopowders, metal nanoparticles, and magnetic fluids offered by Strem is available upon request or via our website. Application sheets discussing the potential use of these products in the medical and pharmaceutical, defense and security, chemical, automotive, and energy fields, and as magnetic fluids, can also be obtained from Strem. More information is also available in the form of a reference sheet listing literature source materials.
| ||||||
Source: Strem Chemicals.
|
 The Opensource Handbook of Nanoscience and Nanotechnology
The Opensource Handbook of Nanoscience and Nanotechnology
 How Nanotechnology Works by Kevin Bonsor and Jonathan Strickland
How Nanotechnology Works by Kevin Bonsor and Jonathan Strickland
 HAPPY NEW YEAR
HAPPY NEW YEAR
 NEWS AS ON Saturday, 8 March 2014
NEWS AS ON Saturday, 8 March 2014
 http://www.crazzyca.blogspot.in/
http://www.crazzyca.blogspot.in/
 10 nano-engineered gadgets to make the trend soon
10 nano-engineered gadgets to make the trend soon
 RISE OF THE MOLECULAR MACHINES
RISE OF THE MOLECULAR MACHINES
 NEWS AS ON Thursday, 10 October 2013
NEWS AS ON Thursday, 10 October 2013
 NEWS AS ON Thursday, 28 November 2013
NEWS AS ON Thursday, 28 November 2013
 Materials Science: The Secret of Nanoparticle Packing in Cement
Materials Science: The Secret of Nanoparticle Packing in Cement
0 comments:
Post a Comment